October 2018
Arun Swaminath, MD, Eric P Berlin, JD, Adam Cheifetz, MD, Ed Hoffenberg, MD, Jami Kinnucan, MD, Laura Wingate, Sarah Buchanan, Nada Zmeter, MD, David T Rubin, MD
Abstract
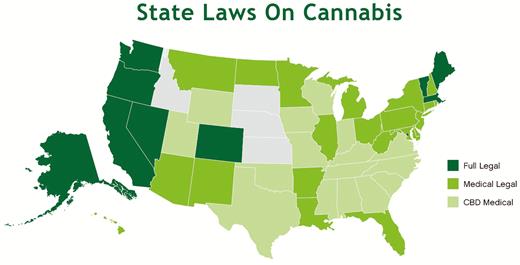
There is significant interest among patients and providers in using cannabis (marijuana) and its derivatives to treat a number of chronic illnesses, including inflammatory bowel disease. Despite the Schedule I classification of cannabis by the federal government, state governments have sought ways to make cannabis available for specific medical conditions, and some states have legalized cannabis outright.
This white paper summarizes the preclinical data, clinical data, safety data, and the regulatory landscape as they apply to medical cannabis use in inflammatory bowel disease. Animal models of cannabinoid chemistry and physiology give evidence of anti-inflammatory, antidiarrheal, and nociceptive-limiting properties. Human studies have found benefit in controlling symptoms and improving quality of life, but no studies have established true disease modification given the absent improvement in biomarker profiles or endoscopic healing.
Finally, this review describes the legal, regulatory, and practical hurdles to studying the risks and benefits of medical cannabis in the United States.